These days, batteries are practically everywhere. In fact, we’ve allowed our society to become totally dependent upon them. They’re so ubiquitous that most people take them for granted. Think about it: Do you really know how a battery works, or do you just count on it without a second thought? Our Battery Depot team wrote this article to address that pesky question of “How do batteries work?”
Before we dive into the specifics, we’ll give you a few hints. Contrary to some popular beliefs:
- It’s not magic.
- It’s not a government conspiracy.
- There’s no pink bunny armed with a bass drum and a pair of retro shades.
How Do Batteries Work: Defining Battery Terms
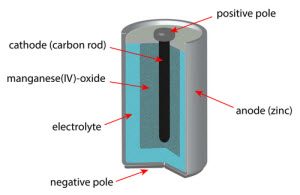 The most important step of any technical explanation of how do batteries work is actually establishing what the terms in question mean. Otherwise, when we say electrolyte, you might think that we’re talking about a red Gatorade; and when we say cathode, you might think that we’re talking about next year’s hottest baby name.
The most important step of any technical explanation of how do batteries work is actually establishing what the terms in question mean. Otherwise, when we say electrolyte, you might think that we’re talking about a red Gatorade; and when we say cathode, you might think that we’re talking about next year’s hottest baby name.
Anode: Negatively charged electrode (metallic conductor) that loses electrons to the cathode.
Cathode: Positively charged electrode (metallic conductor) that accepts electrons from the anode.
Electrolyte: Ionic solution that reacts with the two aforementioned electrodes to give them +/- charges.
Separator: Prevents electrons from flowing inside the battery (short-circuiting); instead, this forces them to flow through the wire (thus creating electricity and powering the attached device).
Electricity: The flow of electrons through a conductive path, such as a wire.
How Do Batteries Work: Chemical Reaction
 And now for the moment we’ve all been waiting for: How do batteries work.
And now for the moment we’ve all been waiting for: How do batteries work.
The two electrodes are situated at opposite ends of a battery. They’re both immersed in an electrolyte fluid. For example, in a typical alkaline battery, the one electrode is comprised of zinc, while the other is comprised of manganese dioxide, and the electrolyte is potassium hydroxide.
This sparks a chemical reaction between the ions of the electrolytes and the metals of each respective electrode: One becomes the negatively charged anode (i.e., it has an excess buildup of electrons), while the other becomes the positively charged cathode (i.e., it has a proportional deficiency of electrons).
This means that there’s now an electrical difference between the anode and the cathode. In other words, the electrons that have built up in the anode want to move toward the electron-starved cathode.
However, there’s a separator to ensure that they don’t make this journey within the battery. By attaching a device to the battery (remember that you attach it at both the negative and the positive terminal), you’re creating a circuit, or, a path through which the electrons can travel from the anode to the cathode.
While they’re on their way, your device basically siphons off a bunch of them as a power source. And there you have it. Voila!
Reach Out to Our Team at BatteryDepot.com for More Information
Phew! That was a lot of info about how batteries work in a short amount of time. If you have any questions, feel free to contact us today.

